Is the Reaction Fe3+ Scn Fescn2+ Endothermic or Exothermic
Based on your observations after heating and cooling the system is the reaction Fe 3 aq SCN - aq FeSCN 2 endothermic or exothermic. Is the reaction between Fe3aq SCN- aq --- FeSCN2aq exothermic or endothermic.

Solved Is The Following Reaction Exothermic Or Chegg Com
As forward reaction is endothermic having a positive rH the reverse reaction is exothermic.

. Click to see full answer. X is the amount of FeSCN2 created determined experimentally. To find the initial concentration of Fe3 use the dilution equation.
Ooops pressed Post too quickly. Ago Bond-breaking is an endothermic process. Explain how you arrived at this answer.
Is the reaction Fe3 aq SCN- aq FeSCN2 aq endothermic or exothermic. See the answer Fe3 aq SCN- aq FeSCN2 aq endothermic or exothermic. First week only 499.
Fe3aq SCNaq FeSCN2aq is exothermic or endothermic. M1V 1V 2 M2 where V2 10 mL. An endothermic reaction an exothermic reaction a system in equilibrium a system that releases heat 2.
Fe3 SCN- -- FeSCN2 original. Think about the net ionic equation. Equilibrium shifts in exothermic reverse direction and the concentration of FeSCN2 will be decreased so colour of solution is lighter.
An SCN- anion interacting with Fe3aq. 2 level 2 Op 5 yr. What does this tell you about the relative strength of an H2O molecule interacting with Fe3aq vs.
KSCN FeNO33 -- FeSCN3 KNO3 21457 results page 10 Chemistry. Weve got the study and writing resources you need for your assignments. What is optimum wavelength.
Fe3 aq SCN- aq FeSCN2 aq Expert Answer 100 3 ratings Fe3 aq SCN- aq FeSCN2 aq Iron III ion and thiocy. Show a sample calculation to help explain this. How do you find the initial concentration of fe3.
Cu2 aq NH3 aq -----. Is the following reaction exothermic or endothermix explain why. Solution for Fe3aq SCNaq FeSCN2aq Is the reaction exothermic or endothermic as written.
Consume more heat if the reaction mixture is heated that is the endothermic reaction is. Based on the following data is this iron thiocyanate reaction endothermic or exothermic. What would happen to the value of K if the reaction were performed at a higher temperature.
Start your trial now. Fe3 aq SCN aq FeSCN2 aq colorless colorless blood-red color After being submreged in a hot water bath the solution was colorless. Our goal in this experiment is to determine the equilibrium constant KcTo do so well need equilibrium concentrations we can.
Write a molecular equation for the precipitation reaction that occurs if any when the following solutions are mixed. Also is FeSCN2 endothermic or exothermic. Le Chateliers Principle predicts that the equilibrium position will shift in order to.
Fe3 SCN- -- FeSCN2 original. What does this tell you about the relative strength of an H 2 O molecule interacting with Fe 3 aq vs. Ammonia reacts with copper II ions Cu2 to form a dark blue copper complex as as shown in the chem equation below.
Yes it is exothermic. The Reaction As Written Is Exothermic. After being submerged in an ice bath the solution turned dark red in color.
Fe3 SCN- FeSCN2 at a specific temperature can be determined by first preparing a standard solution of FeSCN2 and comparing its absorbance of light to an equilibrium Chemistry HCl aq NaOH aq - NaCl aq H2O l Will the neutralisation reaction be endothermic or exothermic. Whether a reaction is endothermic or exothermic depends on the difference between the energy needed to break bondsand the energy released when new bondsform. In an endothermic reaction energy can be considered as a reactant of the reaction.
View the full answer Previous question Next question. If no reaction occurs write NOREACTION. FeSCN2eq Kc ----- Equation 1 Fe3eq SCN-eq whereas described previously brackets denote equilibrium molar concentrations of products reactants.
Temperature depends on the enthalpy of reaction so treat heat as a reactant or product Increase in temperature shift to endothermic reaction Decrease in temperature shift to exothermic reaction Fe3 aq SCN- aqFeSCN2 aq Addition of KSCN crystal shift towards the products salt Addition of Fe NO33 shift towards the right. Obama quickly admits that race is an important part of himself and it is that quest of fe3 scn- fescn2 endothermic race and identity that pushes him to question his own uniqueness. The concentration of the products at equilibrium will be measure by.
Energy is released when new bonds formBond-making is an exothermic process. Use the observations in Part B to identify if the reaction. CuOH2 Cu2 OH- Cu2 OH- You added aqueous ammonia solution NH3 to the equilibrium mixture in test tube 3.
Fe3 SCN- FeSCN2 at a specific temperature can be determined by first preparing a standard solution of FeSCN2 and comparing its absorbance of Chemistry for the reaction 4NH3 g 5O2 g 4NO g 6H2O g if H -950kJmol-1 does that mean its an exothermic reaction. See the answer 1. Is this reaction endothermic or exothermic.
Fe 3 aq SCN - aq FeSCN 2 aq The concentration of one of the ions is altered either by directly adding a quantity of one ion to the solution or by selectively removing an ion from the solution through formation of an insoluble salt. In an exothermic reaction energy can be considered as a product of the reaction. Which of the following needs to remain constant in order for a.
Include a graph of Concentration versus time for this test to show what is happening for this test. The Reaction As Written Is Exothermic. KSCN FeNO33 -- FeSCN3 KNO3.
Fe3 SCN FeSCN2 Rxn 1 Fe3 2SCN FeSCN2 Rxn 2 The nice aspect of this equilibrium is that the reactants are colorless but the product is a deep red color. And SCN - anion interacting with Fe 3 aq. FeSCN2 at equilibrium is determined using Beers Law.
This is an example of. 1 level 1 5 yr.
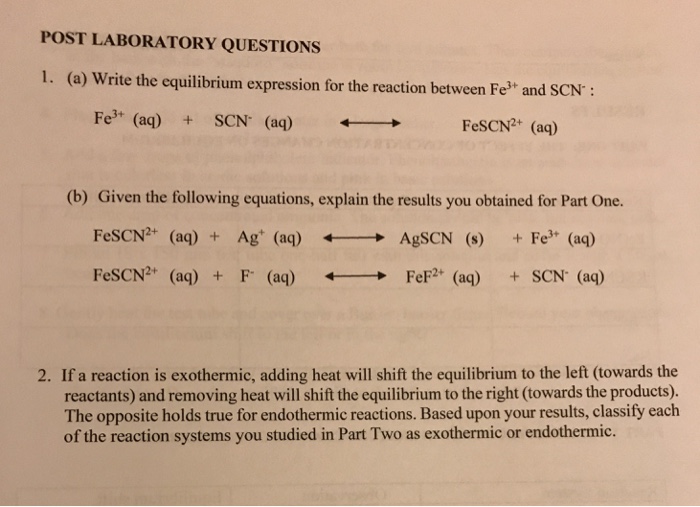
Solved Post Laboratory Questions 1 A Write The Chegg Com

Le Chatelier S Principle Ppt Video Online Download

Solved Based On Your Observations Did The Reaction Fe Chegg Com
Comments
Post a Comment